This post will soon be available in Spanish
opisthoglyphs that use venom that is not life-threatening to humans to subdue their prey (with a decent number of these pending discovery, confirmation, or further investigation). Estimating the percentage of constrictors was more difficult, but I suspected that it was no more than the percentage of snake species that use venom, and probably somewhat less. A lot of people don't realize that there is a huge third category of snakes that just seize their prey and swallow it alive, sometimes subduing it first by crushing it with strong jaws or pinning it to the ground with a coil (which hardly counts as constriction but could be an evolutionary precursor).
This inspired me to do some literature searching, and as I suspected nobody has ever attempted to estimate the exact percentages of snake species that use each kind of prey-killing behavior. As such, I have prepared a preliminary analysis, the full contents of which I intend to make publicly available after peer review. I hope that doing so will stimulate others to publish their observations of feeding behavior in poorly-known snakes (of which there are many), and add to the long history of discussion about the evolution of snake feeding modes, most of which took place before we had a solid grasp on the evolutionary relationships of extant snake families.
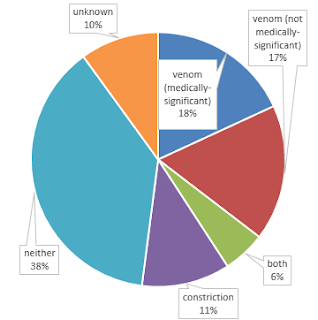
I found that the answer to this question is not as simple as it may seem. Many snakes unambiguously use venom or constriction, but many use neither, and some use both! Of course the data are not as detailed or abundant as we would like. What follows is a break-down of the categories I used, and some interesting exceptions that I uncovered.
Constrictors
Unambiguous constrictors make up just 11% of snake species, but include several well-known groups that are common in the popular consciousness, in zoos, and in the pet trade, including:
- Boas: 61 species, including the eponymous Neotropical Boa constrictor, anacondas (Eunectes), and smaller tree and rainbow boas (Corallus, Epicrates, Chilabothrus) as well as several (sub)families of booid snakes from various and sundry locations around the world—Candoia from New Guinea and Melanesia, sand boas (Eryx) from northeast Africa, the Middle East, and southwestern Asia, Charina and Lichanura from North America, Ungaliophis and Exiliboa from Central America, Acrantophis and Sanzinia from Madagascar, and Calabaria from tropical west-central Africa.
- Pythons: 40 species from Africa, Asia, and Australia
- Ratsnakes, kingsnakes, and close relatives: 43 species of New World colubrine colubrids in the clade Lampropeltini and their Old World counterparts, including:
- Lampropeltis kingsnakes, the consummate constrictors, which prey on other constricting snakes and are rarely, if ever, out-constricted because they are capable of exerting 20 kilopascals of pressure, twice as much as a ratsnake (average 10 kPa)
- southwestern North American species in the genera Pseudelaphe, Arizona, Rhinocheilus, Bogertophis, and Senticolis
- Pantherophis ratsnakes, as well as their sister taxon Pituophis (pine, bull, and gophersnakes), which often press their prey against rocks or other solid objects
- 2 species of live-bearing Eurasian Coronella and their close relative, the Frog-eating Rat Snake Oocatochus rufodorsatus from eastern Russia, Korea, Taiwan, and northeastern China
- 26 species of Old World Elaphe and their relatives in the genera Zamenis, Orthriophis, Oreocryptophis, Euprepiophis, and Archelaphe.
as well as some more obscure groups:
 |
| Anilius scytale constricting an amphisbaenian From Marques & Sazima 1998 |
- Tropidophiids or "dwarf boas", which are not closely related to boids and certainly evolved constriction independently (34 species)
- Their close relative Anilius scytale (sort of; this snake has been observed to constrict large prey such as amphisbaenians)
- Loxocemus bicolor, the Mexican burrowing snake, a close relative of pythons
- Two speceis of Asian sunbeam snakes (genus Xenopeltis), which are also closely related to pythons
- At least some (maybe all) Asian pipesnakes (family Cylindrophiidae)
- Filesnakes (genus Acrochordus), which don't necessarily kill fish by constricting them but use their coils to hold them while they swallow
- some lamprophiine colubrids (especially the well-known African house snakes Lamprophis and Boaedon)
- the colubrine colubrid tribe Lycodontini (mostly wolf snakes, genus Lycodon)
- some snail-eating snakes (Dipsas) coil around snails as they pry them out of their shells
- even Wandering Gartersnakes (Thamnophis elegans)—sometimes! (more below)
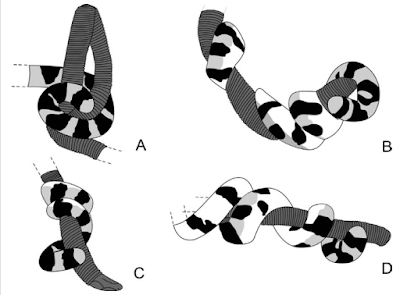
These groups of snakes vary considerably in how often they employ constriction to kill their prey. Some probably use it almost all the time (although even ratsnakes eat prey that they don't constrict, such as bird eggs), whereas others use constriction only rarely, when encountering an unusually large or dangerous prey item relative to their size and strength (for example, one study showed that species of Python, Boa, Pantherophis, and Lampropeltis always constricted mice if they were at least 90% the diameter of the snake's head). Some, such as Regina alleni and Acrochordus filesnakes, may use constriction more so to immobilize the prey than to kill it/it probably doesn’t work that well under water (although Wandering Gartersnakes usually killed mice before eating them).
It seems that mammal-eating is a driver of the evolution of constriction in many cases: species that eat mammals are the only members of their genera/families that use constriction (Thamnophis elegans, Boiga irregularis, Lamprophis/Boaedon, some members of the Oxyrhopus/Clelia/Pseudoboa clade) and both these and species that are nested within mammal-eating clades but have shifted to other prey (Lampropeltis extenuatum, Elaphe quadrivirgata, Cemophora coccinea1) tend to have more variable, less efficient constricting behavior that is generally only used to immobilize rather than to kill prey, if it is used at all. As Alan de Queiroz and Rebecca Groen put it: “Thamnophis elegans are not finely tuned constricting machines” and “Numerous trials in which a garter snake, holding a mouse in its jaws, was chaotically thrown about by the prey's movements support our interpretation that long constriction latencies do not reflect adaptive plasticity in T. elegans.”. Constriction probably functions to reduce the cost of feeding in terms of time, energy, and/or the probability that the prey will harm the snake.
Conspicuously not in this category, we have the poorly-named and misleading North American Racer, Coluber constrictor, which is not a constrictor (thanks for nothing, Linnaeus).
It seems that mammal-eating is a driver of the evolution of constriction in many cases: species that eat mammals are the only members of their genera/families that use constriction (Thamnophis elegans, Boiga irregularis, Lamprophis/Boaedon, some members of the Oxyrhopus/Clelia/Pseudoboa clade) and both these and species that are nested within mammal-eating clades but have shifted to other prey (Lampropeltis extenuatum, Elaphe quadrivirgata, Cemophora coccinea1) tend to have more variable, less efficient constricting behavior that is generally only used to immobilize rather than to kill prey, if it is used at all. As Alan de Queiroz and Rebecca Groen put it: “Thamnophis elegans are not finely tuned constricting machines” and “Numerous trials in which a garter snake, holding a mouse in its jaws, was chaotically thrown about by the prey's movements support our interpretation that long constriction latencies do not reflect adaptive plasticity in T. elegans.”. Constriction probably functions to reduce the cost of feeding in terms of time, energy, and/or the probability that the prey will harm the snake.
Conspicuously not in this category, we have the poorly-named and misleading North American Racer, Coluber constrictor, which is not a constrictor (thanks for nothing, Linnaeus).
 |
| Black Mamba (Dendroaspis polylepis) eating a bird |
- Viperids(341 species), including well-known pit vipers such as rattlesnakes, copperheads, and cottonmouths
- Elapids (359 species), including coralsnakes, cobras, mambas, kraits, sea snakes, and diverse terrestrial Australian snakes ranging from death adders (genus Acanthophis) to bandy-bandys (genus Vermicella)
- Genus Atractaspis (21 species), the stiletto snakes now known to be lamprophiids, which stab backwards with their fangs, mouth closed, to envenomate prey in subterranean burrows
- Front-fanged colubrine colubrids, most notably boomslangs (Dispholidus typus), twigsnakes (genus Thelotornis), and probably their close relatives in the genus Thrasops
- some Asian natricine colubrids in the genera Rhabdophis, Macropisthodon, and Balanophis, which in addition to being (in a few cases lethally) venomous, also have the distinction of being among the only known poisonous snakes
Also, many snakes use venom to subdue their prey but are not dangerous to humans, either because they have fangs in the back of their mouth, have venom that is not adapted for causing physiological damage to mammals, or both. These include:
- numerous dipsadine colubrids from the Caribbean and Central and South America, such as Xenodon, Thamnodynastes, Hydrodynastes, Coniophanes, Erythrolamprus, Rhadinaea, Leptoderia, and Apostolepis (and a few from North America, such as Heterodon and Hypsiglena)
- some colubrine colubrids (genera such as Boiga, Leptophis, Tantilla, Toxicodryas, Platyceps, Oxybelis, Hierophis, Crotaphopeltis, Drymobius, Chilomeniscus, Ficimia, and Gyalopion) as well as the Asian genera Ahaetulla and Chrysopelea, sometimes split into a different subfamily (Ahaetullinae)
- at least some natricine colubrids, such as Paratapinophis praemaxillaris and some North American gartersnakes (Thamnophis)
- many species in the family Homalopsidae,53 species of southeast Asian semi-aquatic snakes, some of which are also well-known for pulling apart large crabs and eating pieces of them
- some (maybe most) lamprophiids, including aparallactines (Amblyodipsas, Aparallactus, Micrelaps, Polemon, Xenocalamus), lamprophiines (Gonionotophis), psamophiines (Mimophis, Psammophis), and the weird genus Psammodynastes ("mock viper")
and probably many more. It's actually possible that this is the largest group, because some of the "unknown" and "neither" species probably actually belong here. An interesting exception are Turtle-headed Seasnakes (Emydocephalus annulatus) and Beaded Seasnakes (Aipysurus eydouxii), which eat fish eggs and have mostly lost their venom, fangs, and venom glands. Another example of a reduction in fangs are some fossorial species of Tantilla, which have only slightly enlarged and faintly grooved rear maxillary teeth, in contrast to the more well-developed rear fangs of most other members of this large genus. These snakes appear to specialize on beetle larvae rather than on centipedes, although no one has looked to see if their venom is any different as a result.
Neither |
| Dipsas indica coiling around a snail, from Sazima 1989 |
- Scolecophidians: Almost 450 species of blindsnakes that eat ant and termite larvae and pupae
- Uropeltids or Shield-tailed Snakes: 55 species that eat almost exclusively earthworms
- Snail-eating snakes in the family Pareidae: 20 southeast Asian species with asymmetrical jaws
- About 300 species of mostly South American dipsadine colubrids that eat soft, gooey things like slugs, snails, earthworms, or frog eggs, including:
- the most speciose genus of snakes, Atractus (currently with 140 species)
- the genus Geophis, currently in a four-way tie for the 5th-most speciose snake genus with 50 species
- some slender arboreal snakes in the genus Sibon, which crawl backward through crevices to wedge snails into them, providing an anchor against which they use their body muscles to pull out the soft parts
- Asian Thermophis
- North American Carphophis and Contia
- Most Old and New World natricine colubrids
- most gartersnakes (Thamnophis)
- watersnakes (Nerodia) and their relatives (Regina, Seminatrix, Clonophis,Tropidoclonion)
- slug-eating Storeria
- Asian ecological analogues (e.g., Amphiesma, Hebius, Opisthotropis, Xenochrophis)
- Numerous colubrine colubrids, such as:
- New World racers and coachwhips (Coluber)
- Indigo snakes (Drymarchon), which are well-known for crushing their prey in their strong jaws
- Indian ratsnakes (Ptyas)
- Egg-eating snakes (Dasypeltis), which have no need to kill the bird eggs that they eat or prevent them from escaping
- Calamariinae, an obscure subfamily of 89 species of colubrids from Asia that are thought to eat mostly earthworms
Some of the aforementioned goo-eaters do use their coils to support the shells of snails while they pry out the soft innards. Dipsas coils around the snail’s shell and Sibynomorphus use as s-shaped loop of their body to support the shell, whereas some Sibon crawl backward through crevices to wedge snails into them, providing an anchor against which they use their body muscles to pull out the soft parts.
Finally, there are some really interesting examples of snakes that use both venom and constriction to subdue their prey, although not always at the same time. Perhaps most impressive but least well-documented in the scientific literature are two viper species that sometimes use constriction in conjunction with venom: Ovophis monticola and O. okinavensis2.
A review by Rick Shine & Terry Schwaner brought together data on numerous Australian elapids that, although they clearly have and use venom, also use their coils to subdue and hold prey while envenoming it. In many of these species, including tiger snakes (Notechis), brown snakes (Pseudonaja), curl/myall snakes (Suta), whip snakes (Demansia), Australian coral snakes (Simoselaps), crowned snakes (Cacophis), and olive seasnakes (Aipysurus laevis), the coils are not used alone as the primary method of prey subjugation, and one recent paper suggested that we think of them as "part of a 'combined arsenal' of prey subjugation strategies".
To explain the "apparent paradox of why a species should use both venom and constriction to subdue its prey", Shine & Schwaner offered three possible non-mutually-exclusive explanations:
 |
| Pseudonaja textilis constricting a mouse From Mirtschin et al. 2006 |
To explain the "apparent paradox of why a species should use both venom and constriction to subdue its prey", Shine & Schwaner offered three possible non-mutually-exclusive explanations:
- The venom may be of low toxicity and thus slow to act, so holding onto the prey with either jaws or coils might allow more venom to be injected
- Species with short fangs, such as Pseudonaja, and/or that feed on on heavily armored prey , such as skinks, may use constriction to give themselves additional time to find a "chink in the armor" and envenomate their prey
- Using constriction in addition to venom may prevent snakes from losing track of bitten and envenomated prey that escape, or from being harmed by retaliating prey that are held onto
- colubrine colubrids Boiga irregularis, Macroprotodon, Platyceps gracilis, Stegonotus, Telescopus, Trimorphodon
- dipsadine colubrids from the Caribbean (Alsophis, Cubophis), Central & South America (Clelia, Helicops, Imantodes, Oxyrhopus, Philodryas, Tropidodryas, Siphlophis, Phimophis, and Pseudoboa), and North America (Diadophis, Farancia)
- the sibynophiine colubrid Sibynophis collaris
- some homalopsids, like Fordonia, Hypsiscopus, and Myron
- a few lamprophiine lamprophiids, such as Lycophidion
- pseudaspine lamprophiids Pseudaspis and Pythonodipsas
- some pseudoxyrhophiine lamprophiids Leioheterodon and Madagascarophis
- some psammophiine lamprophiids (e.g., the Montpellier Snake and its relatives in the genus Malpolon, Hemirhagerrhis, Psammophis, and Rhamphiophis)
- even Wandering Gartersnakes (Thamnophis elegans)—sometimes!
 |
| Elaphe quadrivirgata not constricting a frog (Rana ornativentris) Mori (1991) showed that these snakes constrict large mice, pin small mice with a single coil, and swallow frogs alive |
- Mammals are big, or at least a lot of snakes like to eat mammals that are relatively large compared to their body size
- They are endotherms with the metabolic capacity for sustained struggling
- They can fight back with sharp teeth and strong jaws capable of seriously injuring or killing a snake, in a way that a frog or a lizard cannot
This generalization is supported by observations showing that mammals tend to be killed by constriction prior to being swallowed more often than prey such as frogs, and that larger prey tend to be killed by constriction first, then swallowed. Evidently the amount of struggling is one cue used by Thamnophis elegans to decide whether or not to constrict prey. Experiments carried out by Akira Mori and others have shown that "the degree of such behavioral flexibility is, to some extent, species-specific, and it has been suggested that dietary specialists change their behavior more efficiently than dietary generalists, especially when they are young".
Unknown
After my initial pass at collecting these data (during which I made several sweeping assumptions, some of which later turned out to be oversimplifications), I was left with 36% of species unknown. Following a more thorough literature search, I managed to get this down to 10%, which is still 363 species of snakes. In many cases I made assumptions based on generalizations about the biology of groups of snakes—for instance, I assumed that all scolecophidians use neither constriction nor venom, that all vipers use venom, and so forth. But many dipsadine and colubrine colubrids, and many lamprophiids have not been directly studied, and I could find no reports in the literature about their feeding habits. In some cases we don't even know what they eat, and ecological diversity in these groups is very high, such that there are few consistent patterns that I could use to infer prey subjugation mode for these 370 species. Teach yourself about obscure snakes and help fill in the blanks!
A few examples:
- anomochilids (dwarf pipesnakes)—we don't even know what they eat
- many Malagasy pseudoyrhophiine lamprophiids (e.g., Ithycyphus, Alluaudina)
- lamprophiids from mainland Africa, such as Bothrolycus, Chamaelycus, and Dendrolycus
- obscure dipsadines—e.g., Cryophis, Tantalophis
- obscure colubrines—e.g., Bamanophis, Hemerophis
- most of the xenodermids (Fimbrios, Parafimbrios, Stoliczkia, Xylophis)
- species known only from a few specimens, like Xenophidion
 |
| Phylogenetic tree from Greene 1994 For an overview of some of the updates, click here |
The most recent similar review was done by Harry Greene in 1994, in which he revised earlier hypotheses he put forth with Gordon Burghardt in the journal Science 16 years before. We now know a lot more about the snake family tree than we did in 1994, particularly the fine details of relationships within the Caenophidia. Overall, the basic pattern has held up rather well—constriction evolved first in basal alethinophidians during the late Cretaceous, accompanying or preceding most other evolutionary innovations that permit snakes to consume large prey, such as kinetic skulls. Greene pointed out that this was before the origin of rodents, often mentioned as potentially relevant to the evolution of snake prey-killing behaviors. Constriction was then lost at least twice—once in uropeltids (which feed underground on earthworms, although I'm not actually aware of any detailed observations of uropeltid feeding behavior) and at least once in basal colubroids, where it might have been at first replaced by venom. Venom was then subsequently lost in numerous caenophidian lineages, replaced by re-evolution of constriction in some or by other specializations (tooth diastemata for holding skinks, egg-eating) in others, and in some caenophidian lineages snakes use both as appropriate, sometimes together (or they may elect to use neither even if both are available).
Both constriction and venom reduce the cost of feeding in terms of time, energy, and/or the probability of the prey harming the snake, but in constricting snakes, everyday locomotion and large prey neutralization are coupled, whereas in venomous snakes they are independent (snakes don't use their fangs to get around). This could be one reason why venom as an evolutionary innovation led to a more speciose radiation of snakes; it's also more susceptible to evolutionary arms races, because prey can evolve resistance to certain venom compounds, but not to constriction. Specialization for constriction is more than just behavior—constricting species also have more vertebrae per unit length than non-constricting species. And there are costs to both, which must be outweighed by the benefits of that defining snake trait: being able to consume prey almost as large, and sometimes much larger, than yourself!
1 An interesting exception are Scarletsnakes, Cemophora coccinea, the closest relatives of kingsnakes, which feed mostly on reptile eggs but also use their coils to hold lizard prey in the rare instances when they eat them. It is certain that Scarletsnakes evolved from constricting ancestors but because they almost never eat prey that need to be killed beforehand, evidently they rarely constrict.↩
2 okinavensis has been shown not to be closely related to other Ovophis, but no new genus has yet been created for it because more data are needed.↩
ACKNOWLEDGMENTS
Thanks to Karen Morris for asking me this question, and to Alpsdake and Danny Davies for the use of their photos.
SELECTED REFERENCES
For a full list of all the references I consulted in preparing this post, click here
Andrade, R. d. O. and R. A. M. Silvano. 1996. Comportamento alimentar e dieta da "Falsa-coral"Oxyrhopus guibei Hoge & Romano (Serpentes, Colubridae). Revista Brasileira de Zoologia 13:143-150 <full-text>
Auffenberg, W. 1961. Additional remarks on the evolution of trunk musculature in snakes. The American Midland Naturalist 65:1-16 <full-text>
Bealor, M. T. and A. J. Saviola. 2007. Behavioural complexity and prey-handling ability in snakes: gauging the benefits of constriction. Behaviour 144:907-929 <ResearchGate>
Bealor, M. T., J. L. Miller, A. de Queiroz, and David A. Chiszar. 2013. The evolution of the stimulus control of constricting behaviour: inferences from North American gartersnakes (Thamnophis). Behaviour 150:225-253 <full-text>
de Queiroz, A. and R. R. Groen. 2001. The inconsistent and inefficient constricting behavior of Colorado western terrestrial garter snakes, Thamnophis elegans. Journal of Herpetology 35:450-460 <full-text>
Franz, R. 1977. Observations on the food, feeding behavior, and parasites of the striped swamp snake, Regina alleni. Herpetologica 33:91-94 <full-text>
Gans, C. 1976. Aspects of the biology of uropeltid snakes. Pages 191-204 in A. d. A. Bellairs and C. B. Cox, editors. Morphology and Biology of Reptiles. Linnean Society Symposium Series No.3. Academic Press, London.
Götz, M. 2002. The feeding behavior of the snail-eating snake Pareas carinatus Wagler 1830 (Squamata: Colubridae). Amphibia-Reptilia 23:487-493 <ResearchGate>
Götz, M. 2002. The feeding behavior of the snail-eating snake Pareas carinatus Wagler 1830 (Squamata: Colubridae). Amphibia-Reptilia 23:487-493 <ResearchGate>
Greene, H. W. 1994. Homology and behavioral repertoires. Pages 369-391 in B. Hall, editor. Homology: The Heirarchical Basis of Comparative Biology. Academic Press, San Diego <Google book>
Greene, H. W. and G. M. Burghardt. 1978. Behavior and phylogeny: constriction in ancient and modern snakes. Science 200:74-77 <abstract>
Hampton, P. M. 2011. Ventral and sub-caudal scale counts are associated with macrohabitat use and tail specialization in viperid snakes. Evolutionary Ecology 25:531-546 <link>
Holm, P. A. 2008. Phylogenetic biology of the burrowing snake tribe Sonorini (Colubridae). PhD dissertation. University of Arizona <full-text>
Jackson, K. and T. H. Fritts. 2004. Dentitional specialisations for durophagy in the Common Wolf snake, Lycodon aulicus capucinus. Amphibia-Reptilia 25:247-254 <full-text>
Loop, M. S. and L. G. Bailey. 1972. The effect of relative prey size on the ingestion behavior of rodent-eating snakes. Psychonomic Science 28:167-169 <full-text>
Hampton, P. M. 2011. Ventral and sub-caudal scale counts are associated with macrohabitat use and tail specialization in viperid snakes. Evolutionary Ecology 25:531-546 <link>
Holm, P. A. 2008. Phylogenetic biology of the burrowing snake tribe Sonorini (Colubridae). PhD dissertation. University of Arizona <full-text>
Jackson, K. and T. H. Fritts. 2004. Dentitional specialisations for durophagy in the Common Wolf snake, Lycodon aulicus capucinus. Amphibia-Reptilia 25:247-254 <full-text>
Loop, M. S. and L. G. Bailey. 1972. The effect of relative prey size on the ingestion behavior of rodent-eating snakes. Psychonomic Science 28:167-169 <full-text>
Marques, O. A. V. and I. Sazima. 2008. Winding to and fro: constriction in the snake Anilius scytale. Herpetological Bulletin 103:29-31 <link>
Martins Teixeria, D., M. Luci Lorini, V. G. Persson, and M. Porto. 1991. Clelia clelia (Mussurana). Feeding behavior. Herpetological Review 22:131-132 <link>
Martins Teixeria, D., M. Luci Lorini, V. G. Persson, and M. Porto. 1991. Clelia clelia (Mussurana). Feeding behavior. Herpetological Review 22:131-132 <link>
Mehta, R. S. and G. M. Burghardt. 2008. Contextual flexibility: reassessing the effects of prey size and status on prey restraint behaviour of macrostomate snakes. Ethology 114:133-145 <full-text>
Mirtschin, P. J., N. Dunstan, B. Hough, E. Hamilton, S. Klein, J. Lucas, D. Millar, F. Madaras, and T. Nias. 2006. Venom yields from Australian and some other species of snakes. Ecotoxicology 15:531-538 <full-text>
Mori, A. 1991. Effects of prey size and type on prey-handling behavior in Elaphe quadrivirgata. Journal of Herpetology 24:160-166 <link>
Mori, A. and K. Tanaka. 2001. Preliminary observations on chemical preference, antipredator responses, and prey-handling behavior of juvenile Leioheterodon madagascariensis (Colubridae). Current Herpetology 20:39-49 <full-text>
Mushinsky, H. R. 1984. Observations of the feeding habits of the short-tailed snake, Stilosoma extenuatum in captivity. Herpetological Review 15:67-68 <link>
Mirtschin, P. J., N. Dunstan, B. Hough, E. Hamilton, S. Klein, J. Lucas, D. Millar, F. Madaras, and T. Nias. 2006. Venom yields from Australian and some other species of snakes. Ecotoxicology 15:531-538 <full-text>
Mori, A. 1991. Effects of prey size and type on prey-handling behavior in Elaphe quadrivirgata. Journal of Herpetology 24:160-166 <link>
Mori, A. and K. Tanaka. 2001. Preliminary observations on chemical preference, antipredator responses, and prey-handling behavior of juvenile Leioheterodon madagascariensis (Colubridae). Current Herpetology 20:39-49 <full-text>
Mushinsky, H. R. 1984. Observations of the feeding habits of the short-tailed snake, Stilosoma extenuatum in captivity. Herpetological Review 15:67-68 <link>
Penning, D. A. and B. R. Moon. 2017. The king of snakes: performance and morphology of intraguild predators (Lampropeltis) and their prey (Pantherophis). The Journal of Experimental Biology 220:1154 <link>
Rossi, J. V. and R. Rossi. 1993. Notes on the captive maintenance and feeding behavior of a juvenile short-tailed snake (Stilosoma extenuatum). Herpetological Review 24:100-101 <link>
Savitzky, A. H. 1980. The role of venom delivery strategies in snake evolution. Evolution 34:1194-1204 <link>
Sazima, I. 1989. Feeding behavior of the snail-eating snake, Dipsas indica. Journal of Herpetology 23:464-468 <link>
Sazima, I. 1989. Feeding behavior of the snail-eating snake, Dipsas indica. Journal of Herpetology 23:464-468 <link>
Shine, R. 1977. Habitats, diets, and sympatry in snakes: a study from Australia. Canadian Journal of Zoology 55:1118-1128 <abstract>
Shine, R. and T. Schwaner. 1985. Prey constriction by venomous snakes: a review, and new data on Australian species. Copeia 1985:1067-1071 <link>
Stettler, P. H. 1959. Zur Lebensweise von Dipsas turgidus (Cope), einer schneckenfressenden Schlange. Aquarien und Terrarien 8:238-241.
Stettler, P. H. 1959. Zur Lebensweise von Dipsas turgidus (Cope), einer schneckenfressenden Schlange. Aquarien und Terrarien 8:238-241.
Vidal, N. and S. B. Hedges. 2002. Higher-level relationships of snakes inferred from four nuclear and mitochondrial genes. Comptes Rendus-Biologies 325:977-985 <link>
Willard, D. E. 1977. Constricting methods of snakes. Copeia 1977:379-382 <link>
Life is Short, but Snakes are Long by Andrew M. Durso is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.